Our group is interested in the chemical properties of heavy elements with Z ≥ 104. These artificial elements usually consist of short-lived nuclides. Thus, our goal is to develop chemical procedures aimed at fast and selective isolation of a desired radioactive element and to understand the mechanism of the element’s behavior under given conditions. First, we focus on lighter homologs of heavy elements. Despite that they do have stable nuclides, we intentionally work with commercially available medical isotopes to substantially increase the sensitivity of our methods. These extremely low concentrations are needed to mimic the “atom-at-a-time” nature of transactinide experiments, where there is never more than one atom present at a time. We start our investigations by applying wet chemistry techniques along with gas phase chemistry at a later stage. The novelty of our studies is based on the application of a new class of compounds that has been barely utilized (if used at all) by the heavy elements community. These compounds are organic salts, consisting of discrete cations and anions, with a melting point below 100 °C. However, the majority of them have a melting point below room temperature, and that is why they are called ionic liquids. If an ionic liquid is water immiscible, then a two-phase system is formed and partitioning of an element of interest between the two phases can be studied (see Fig. 1).
![Thallium Extraction with [C4mim][Tf2N]](https://cyclotron.tamu.edu/folden/wp-content/uploads/sites/2/Thallium-Extraction-with-C4mim-Tf2N-v1-1024x789.png)
So far, our work has been focused on studying indium and thallium behavior towards understanding potential nihonium (element 113) chemistry. For example, we have just finished a full characterization of a betainium-based ionic liquid and its role in both indium and thallium distribution in hydrochloric acid media (see Fig. 2). We have developed mechanisms for the extraction, and we are using this to design systems that will potentially interact with heavy elements in a controlled way.
![[HBet][Tf2N] Extraction Scheme](https://cyclotron.tamu.edu/folden/wp-content/uploads/sites/2/HBetTf2N-Extraction-Scheme.png)
The ability to tune the properties of ionic liquids (a form of “designer solvents”) also includes polymerization of any ion involved with formation of a solid film (see Fig. 3). This changes the interaction from liquid-liquid to liquid-solid. For example, the polymer can potentially be used to coat any surface forming a chromatography column (see Fig. 4). It has been shown by our group that such a polymer still has chemical selectivity and can extract metals of interest from aqueous solutions.
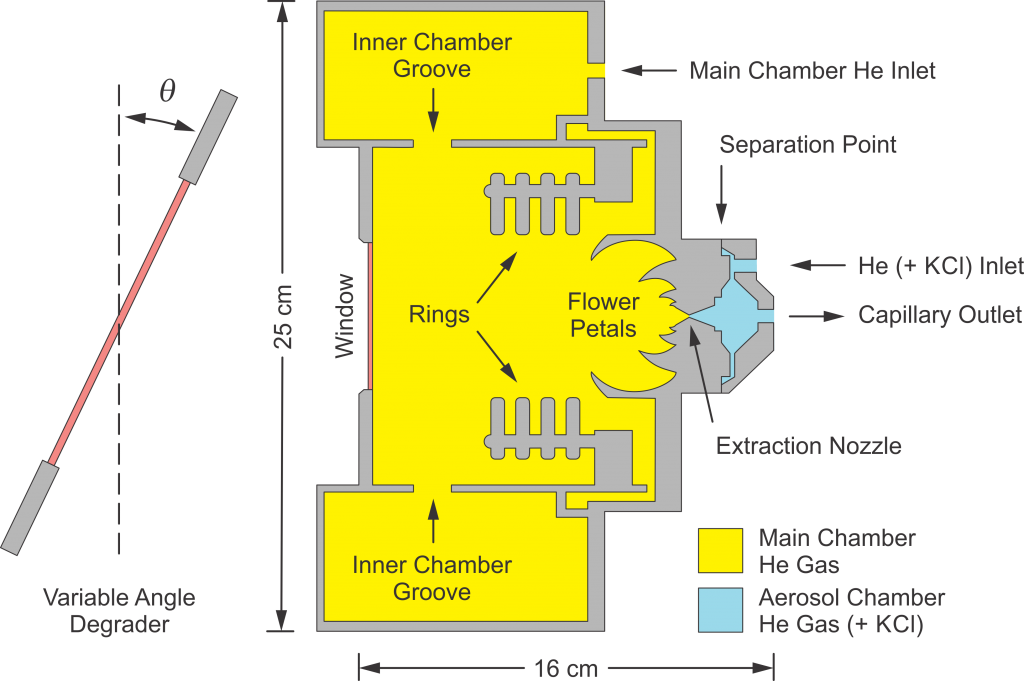
Another interesting direction is the replacement of discrete ions with Lewis acids and bases according to their hydrogen bonding. These hydrogen bond donor-acceptor mixtures possess unique properties, and the most characteristic one is a decrease in mixture melting temperature. Thus, two or more solids at room temperature can be combined with an appropriate ratio between the initial compounds, resulting in a liquid water-immiscible eutectic mixture. Our group was the first in the world to demonstrate the suitability of such mixtures for the metals extraction. Our medium-term goal is to start performing “online” chemistry gas-phase experiments, where radioactive material is produced in a nuclear reaction and used within seconds of production. We have designed and characterized a cutting-edge “gas stopper” that will be used to “thermalize,” or slow down, heavy atoms produced in nuclear reactions to the energy necessary for chemical experiments. This gas stopper will be used to deliver these products to a new chemistry laboratory where we will measure their chromatographic properties, and is described in a recent paper. A schematic of the gas stopper is shown in Fig. 5. These experiments will provide information on the complexation behavior of these elements, and will help determine whether trends in the periodic table are maintained for the heaviest elements, since there are reasons to believe that the periodicity of the elements may not hold for extremely high atomic numbers.